The Wright group is interested in applying our mixed electronic-vibrational pathways to protein metal centers in order to better understand their catalytic activity. These pathways include Doubly Vibrationally Enhanced (DOVE) and Triple Sum Frequency (TSF, TRSF) Four-Wave Mixing. Each of these pathways (thus far) probes two vibrational states and one electronic state. By matching the visible w3 frequency to the electronic transition of the moiety of interest, its signal will rise above the background much like in resonance Raman. In this case, however, two remaining frequencies are left to probe coupling between vibrational modes as one does in 2D-IR. This opens a new avenue for understanding structures and dynamics that may be difficult to probe with other methods.
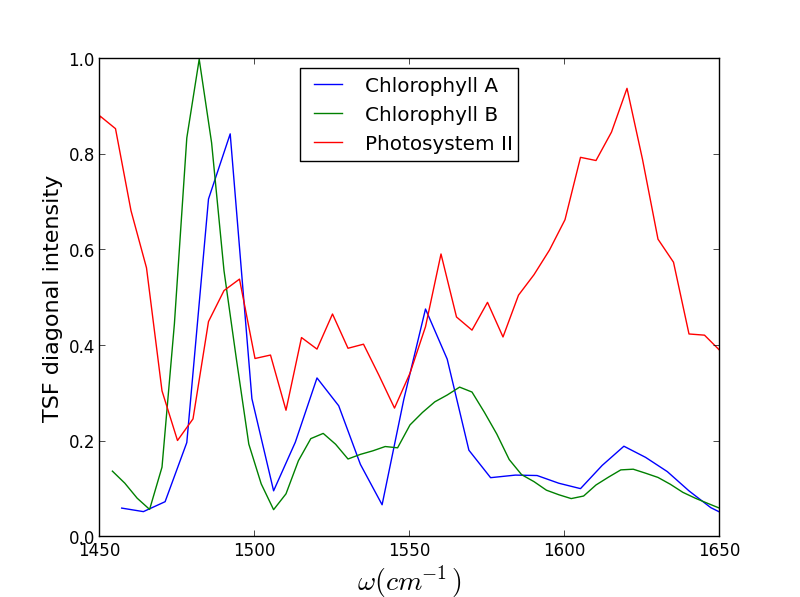
We have recently been exploring the ability for Triply Resonant Sum Frequency Spectroscopy (TRSF) to obtain 2D vibrational spectra of components within proteins while excluding the protein background. Though we do not yet have data for a metal center, we have witnessed chlorophyll spectra rising above the protein background, giving promise to further applications. On the left below is the TRSF spectrum of a photosystem II core complex (care of the Brudvig group at Yale). The right two TRSF spectra are Chlorophyll a and b, respectively. They are quite similar since the two species have only one functional group different -- a carbonyl off the porphyrin ring becomes a methyl group.
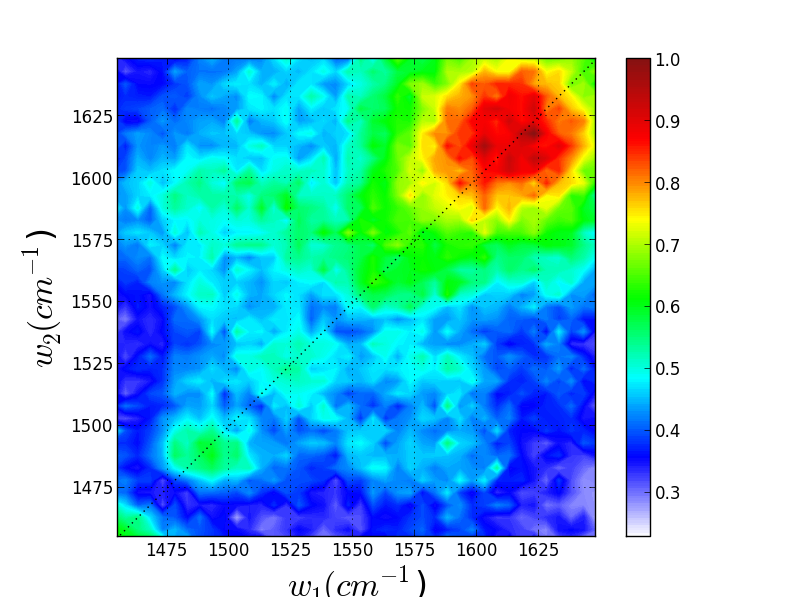
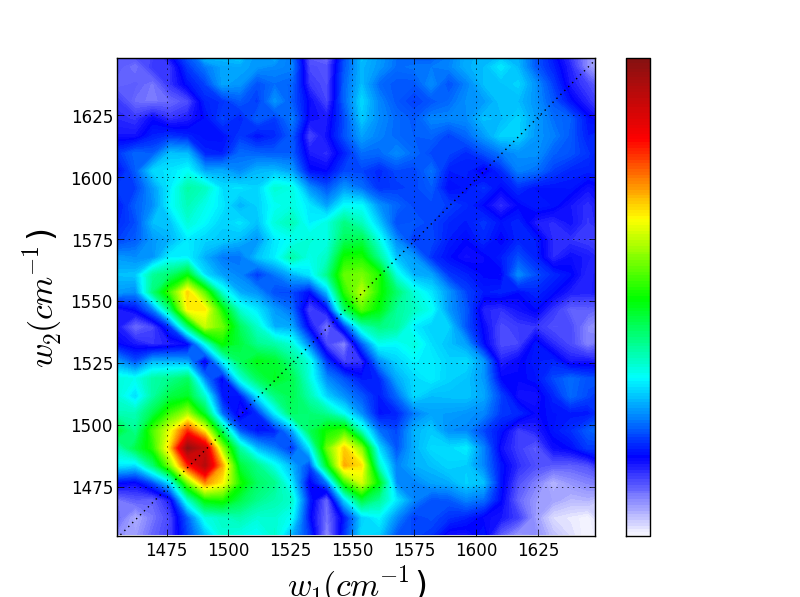
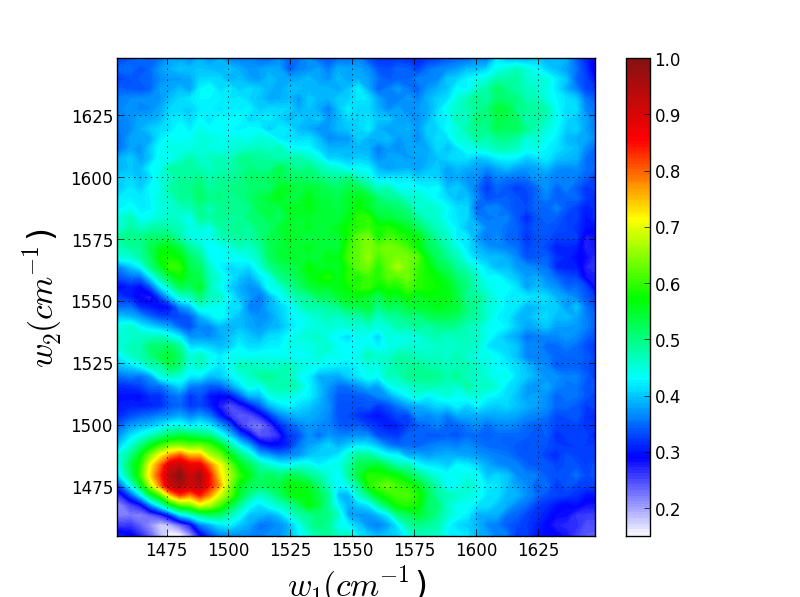
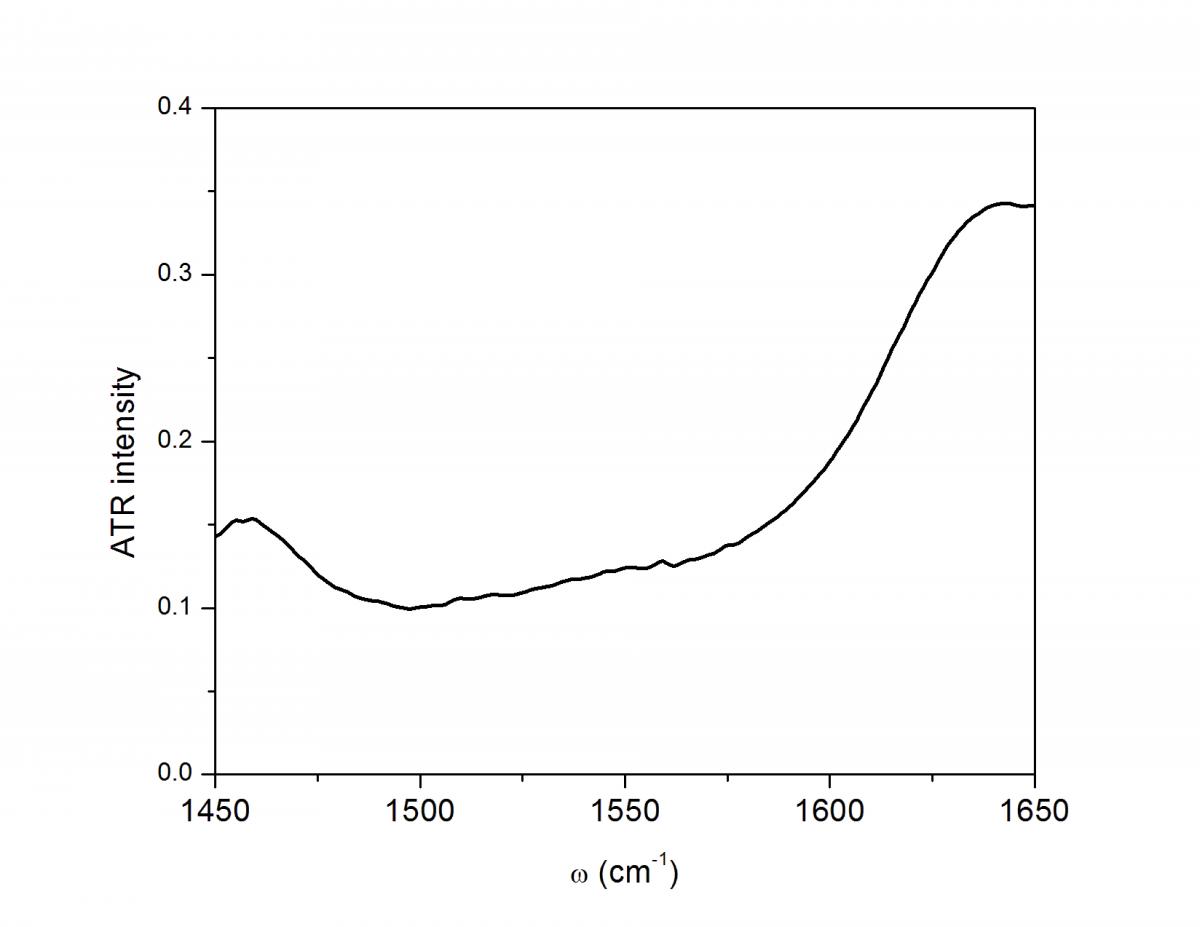
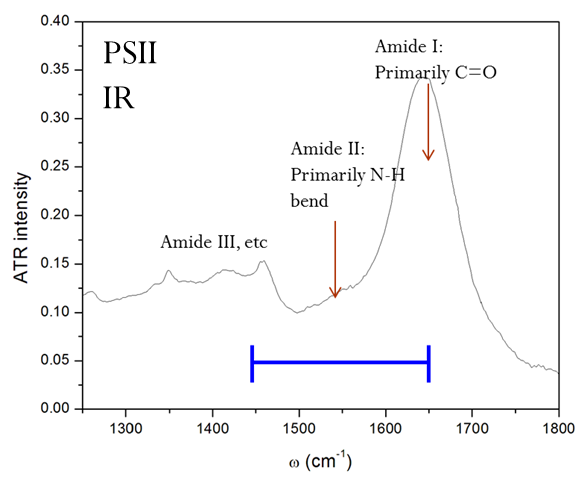
Though these 3 TRSF spectra are noticably different, a diagonal slice through them shows clear correlation in the peak frequencies (lower left spectrum). This indicates that we are indeed picking chlorophyll out of the enormous protein background. This is especially evident if you look at the IR spectrum of PSII, which has the usual amide modes observed in proteins far outcompeting the chlorophyll modes observed in TRSF.